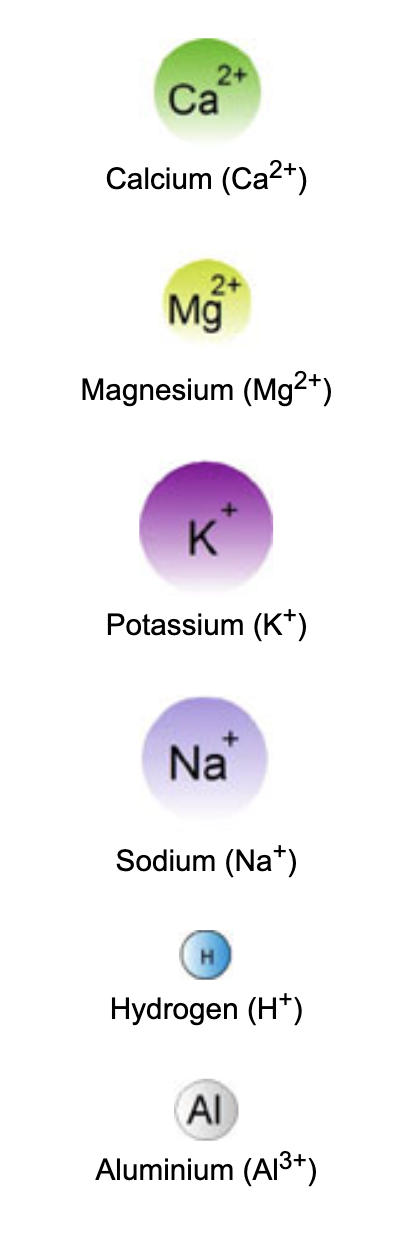
Exchangeable Cations
Source: http://www.terragis.bees.unsw.edu.au/terraGIS_soil/sp_exchangeable_cations.html
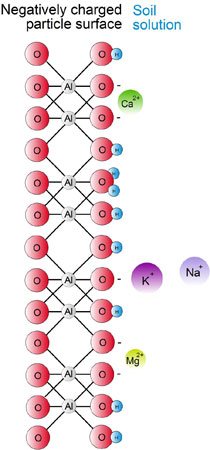
The surface of an individual clay particle or organic colloid is negatively (-) charged. As a consequence their surfaces attract and adsorb positively charged ions called cations. When water is added to soil, cations can move into solution, however, they are still attracted to the clay particle or organic colloid surface and as a result swarm around them.
The mechanism of adsorption and desorption is important, even though less than 1 % of cations will do this at any one time. This is because detached cations become available to plants. Conversely, when they are adsorbed to soil particles, the leaching of these plant available nutrients is reduced.
Positively charged ions capable of being readily substituted from the soil solution and onto the surface of a negatively charged soil particle, and vice-a-versa, are termed exchangeable cations. The exchangeable cations of most importance are;
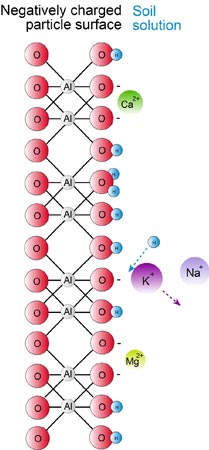
In order to become available to a plant, a cation adsorbed on a clay particle must be replaced by a cation present in the soil solution. Plant roots facilitate this process by excreting a hydrogen ion (H+) into the soil solution in order to exchange this for a cation (e.g. potassium – K+).
The cumulative effect of a hydrogen (H+) ion being given up by a plant leads to soil acidification if the cation (e.g. K+) is not replaced. In this way the introduction of H+ ions into the soil solution also increases the rate of weathering of primary- (e.g. feldspars) and secondary clay-minerals (e.g. kaolin).
The process of substitution is known as cation exchange and occurs only when a cation in the soil solution moves into the hemisphere of motion of a cation located on the surface of a negatively charge particle. This is shown diagrammatically, for the potassium ion (K+), which is exchanged from the surface of a clay particle by the hydrogen ion (H+) which moves from the soil solution.
Cation exchange is therefore defined as the interchange between a cation on the surface of any negatively charged particle (i.e. clay mineral or organic colloid) and the soil solution. Whilst the cations themselves are still attracted to the clay particle, the force of attraction on the cations diminishes rapidly with increasing distance from the negatively charged surface.
This phenomenon of attracted cations and negatively charged particles is known as the ‘diffuse double layer’. Literally it is a “double layer” because there are two layers of charge (i.e. negative and positive) and “diffuse” because the outer layer of cations is not well defined. The force of attraction between the negatively charged particles and the cations reduces quickly with increasing distance.
Apart from the plant nutritional aspects of cations, adsorbed cations also influence the behavior of clay soil. This is because the binding force of individual cations is a function of various factors, including;
- Cation charge (i.e. valence),
- Size of hydrated cation (i.e. ionic radius), and
- Concentration of charge, and
- Thickness of the double layer outside the surface of clay particles.
In the first instance, the more strongly attracted a cation is to the exchange surface, the greater is the chance of adsorption. This is known as the energy of adsorption. The energy of adsorption of a cation is a function of the valence (i.e. charge). This is the reason why trivalent (+3 charge) cations such as aluminium and divalent (i.e. +2 charge) cations such as calcium and magnesium, have an energy of adsorption respectively, almost three and two times that of monovalent (i.e. +1 charge) cations such as potassium or sodium. As a consequence an exchangeable cation of aluminum, calcium or magnesium stays close to the clay particle and does not interfere with the cohesion between aggregated particles. In fact these cations initiate the process of particle aggregation in soil.
The valence of an exchangeable cation therefore determines the double layer thickness. So that, the higher the valence of the dominant exchangeable cation, the thinner the double layer. However, differences in the thickness of the double layer can still occur even when the cations are of equivalent charge or valency.
When the valence of the cations are equal (i.e. both +1 charge) the cation with the smallest hydrated radius is more strongly adsorbed. In the case of the monovalent cations of potassium and sodium, the potassium ion is more strongly adsorbed since it has a smaller hydrated radius and hence is more strongly adsorbed to the site of the negative charge. In comparison the sodium ion is so loosely held and so ready to hydrate that sodium rich soil will disperse.
|
Radius |
Unit |
Na+ |
K+ |
Mg2+ |
Ca2+ |
Al3+ |
|
Non-hydrated |
nm |
0.095 |
0.133 |
0.066 |
0.099 |
0.050 |
|
Hydrated |
nm |
0.360 |
0.330 |
0.430 |
0.410 |
0.480 |
This is similarly the case with the divalent cations of calcium and magnesium. Because the hydrated magnesium ion is larger than that of calcium, the magnesium ion is held more weakly and behaves in some instances in soil (i.e. when calcium is low) like sodium.
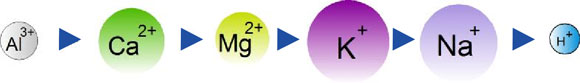
The charge of the cation and the size of the hydrated cation essentially govern the preferences of cation exchange equilibria. In summary, highly charged cations tend to be held more tightly than cations with less charge and secondly, cations with a small hydrated radius are bound more tightly and are less likely to be removed from the exchange complex. The combined influence of these two criteria can be summarized generally by the lysotrpoic series.
Aluminium > Calcium > Magnesium > Potassium, Ammonium-NH4+ > Sodium > Hydrogen
It indicates, from left to right, the decreasing strength of adsorption of the various cations. As such, the less tightly held cations are located furthest from the surface of colloids and are most likely to be leached away or further down the profile most quickly. Conversely, the most strongly adsorbed cations will tend to move the slowest down through the profile.
The proportion and kinds of cations adsorbed on soil mineral particles and organic colloids is also a function of the concentration of cations in the soil solution. If the concentration of a cation in soil solution is high, there is an increased chance or tendency for that cation to be adsorbed.
This is the reason that dissolved gypsum (CaSO4) is added to ameliorate sodic soil. In this case, the addition of dissolved gypsum increases the concentration of calcium in the soil solution and this leads to an increase in calcium ions on the exchange complex at the expense of exchangeable sodium.
The major source of cations in soil solution are from mineral weathering (i.e. primary minerals), mineralization of organic matter and addition of soil ameliorants (i.e. lime, gypsum, etc).